radio mycelium
A mycelial network has no organs to move the world, no hands; but higher animals with manipulative abilities can become partners with the star knowledge within me and if they act in good faith, return both themselves and their humble mushroom teacher to the million worlds all citizens of our starswarm are heir to.
[Terence McKenna. The Mushroom Speaks]
The mycelium, a fungal network of thread-like cells, represents a truly underground communications network, spreading out over vast areas of earth substrate, acting with ecosystem intelligence as an interface across symbiotic networks such as plant and tree roots.
The Radio Mycelium workshop aims to actively examine the cross-spore-germination between two parallel wide area networks; between radio-based communication technologies and the single organism network of the mycelium. Fungal transceivers sprouting mycelial antennas form an imaginary underground network. Diversity of human networks is mapped across fungal diversity in the urban environment. The influence of electromagnetic carrier wave on the mycelial network is to be examined.
The well-documented transformative potential of the mycelium (for example, decomposing pollutants) is invoked to remediate an increasingly pathogenic electromagnetic networked culture.
A series of experimental situations will be established and studied by participants, who will also learn how to construct simple measurement devices, and culture shiitake, pink oyster and Enokitake mushrooms, amongst other mycellium (simple moulds). A short field trip will find and map winter mushroomings of electromagnetic fungi in Brussels.
radio mycelium is supported by foam: http://fo.am/radio_mycelium/ as part of http://fo.am/parn.
Schedule
day one:
10:00 intro Christina - with time overview of the 2 days
10:15 intro Martin, and overview contents
10:45 intro round of participants and discussion (interests, thoughts)
11:15 construction of apparatus for experimental setups (sterile box for culturing some spores I collected onto agar, and some magnetic field amplifiers: LRA design below and field amp for walk)
13:00 lunch
14:30 - set up of experiments (including HOWTOs for growing mushrooms and - moulds) - mycelial measurements (overview of what could be measured between - mushrooms and magnetic fields)
17:00 participating mushroom practitioners give overviews on their projects and recent research
dinner at “le cafe des spores”
day two:
09:45- 11:45 outdoors - mushroom measurement walk combined with - guerilla spawning in the city
12:00 back indoors: re-focus on experiments and examine all results/logs
13:00 - 14:30 lunch
14:30 - discussion with focus on outcomes (how to!) - here we should get some input for a possible manual (tutorial aspect)
16:30 future projects and rounding up 18:00 end
notes: If inoculating away from electricity, using a camp stove, make sure that the wax is hot when you apply it; otherwise, the wax will not create a tight seal and can easily fall off. The wax can be applied with a foam paint brush or cotton dauber.
References
General cultivation: http://reallyrandomgarden.blogspot.com/2011/02/mushroom-growing.html
Complete guide to cultivation: http://www.mykoweb.com/articles/cultivation.html
Influence of ELF sinusoidal electromagnetic fields on proliferation and metabolite yield of fungi: http://www.ncbi.nlm.nih.gov/pubmed/16595336
http://www.automenta.com/mycocomputer
http://abject.ca/do-trees-communicate/
the Language of Mycorrhizal Communication: http://cymeandcystidium.com/?p=135
http://www.shiitake.de/eng/an_intro/an_intro.html - also as supplier in Germany of inoculated substrates
Tissue culture: http://omnisterra.com/botany/cp/slides/tc/tc.htm
oysters on cardboard: http://www.redoakmushroom.com/
Further notes, experiments and research
Experiments towards the setting up of a temporary psycho-mycological lab:
Bread moulds
Simply adding a few drops of water to a piece of roggenmischbrot, leaving it in the open air for one hour and then placing in an airtight plastic container at room temperature. After four days (11th to 15th November we can see seven patches of white mould.
After six days (11th to 17th):
Construction of sterile/still air glove box
Upturned clear plastic storage container with two holes for arms (could use heated metal coffee can to make these holes). In this case we just heated up a craft knife with a lighter and cut the holes in sections. The arm holes were covered with microfase dishcloth material.
To be sprayed down with 10% bleach solution and/or cleaned with alcohol before use.
Refs:
https://mycotopia.net/archives/discus/messages/5/1399.html?998567608
http://www.thetaobums.com/index.php?/topic/20968-purple-reishi/
Sterile/cleanliness notes
Tape down plastic sheet over table (in a location away from draughts, plants, pets and people) and clean the sheet with bleach/alcohol. Water spray in the air beforehand.
Wash hands and nails with antibacterial soap, hair tied back or wear hat.
Use 80% isopropyl alcohol on hands every few minutes.
Are dishes totally sealed or with some kind of filter? We seal them with sellotape.
How are the (non-sterile) plastic petri-dishes sterilised? By bleaching or steaming: http://www.ehow.com/how_5892646_sterilize-petri-dishes.html
Recipes for agar mycelium growth
- Malt Extract, Yeast Agar:
1litre water, 20g agar agar, 20g barley malt/malt extract
Add these ingredients to one liter of boiling water. After they have dissolved it is ready to be poured.
(or mix 1 tbsp agar in 1 cup water, low boil and simmer, stirring, add pinch of nutrient)
- Potato, Dextrose, Yeast Agar:
1litre water, 300g potato water (broth from boiling potatoes for 1 hour), 20g agar agar, 10g dextrose, 2g yeast
Shred the unpeeled potatoes into a colander and then rinse them for thirty seconds with cold tap water. Combine the rinsed potatoes with one liter of water and gently boilf for 30 minutes. Filter the resulting potato broth through muslin or cheescloth and discard the potatoes. To the liter of potato broth add the agar, dextrose and yeast which you have previously weighed out, mixed together, and set aside. While stirring gently add in the mixed powdered indgredients. Gently boil for ten minutes or until the solution is clear. Take care to not allow the solution to boil over. Add enough water to return the total volume of the solution to one liter. Pour the solution while still hot and liquid into petri plates, baby food jars, or slant culture tubes. Use just enough to cover the bottom of the containers, about 1/4 inch deep or a little more. The solution may be allowed to cool or sterilized immediately.
Do we need a pressure cooker for agar? or see MAKE article just boil agar: http://makeprojects.com/Project/Home-Mycology-Lab/242/1
Refs:
http://www.shroomery.org/8510/A-document-about-agar-from-Lycaeum
http://www.articleclick.com/Article/Growing-Mushrooms-from-Home-using-Agar-Plates/2218
http://www.mrcashop.org/mushroom_shop/index.php?content=AgarPreperation
growing mycel with spores
Spores are collected by making a spore print; the mushroom cap is seperated and placed gills down on a piece of paper. A container can be placed over the ensemble. Spore prints could also be made on glass (Stamets).
Procedure:
- Flame sterilise inoculation loop (home made with 1mm wire and small loop+gaffer tape) or scalpel
- Cool tip in the petri dish containing medium
- Pick up spores from print using the loop
- Streak the tip of the loop across the dish
documentation:
Process above was followed on 19.11.11 with following notes:
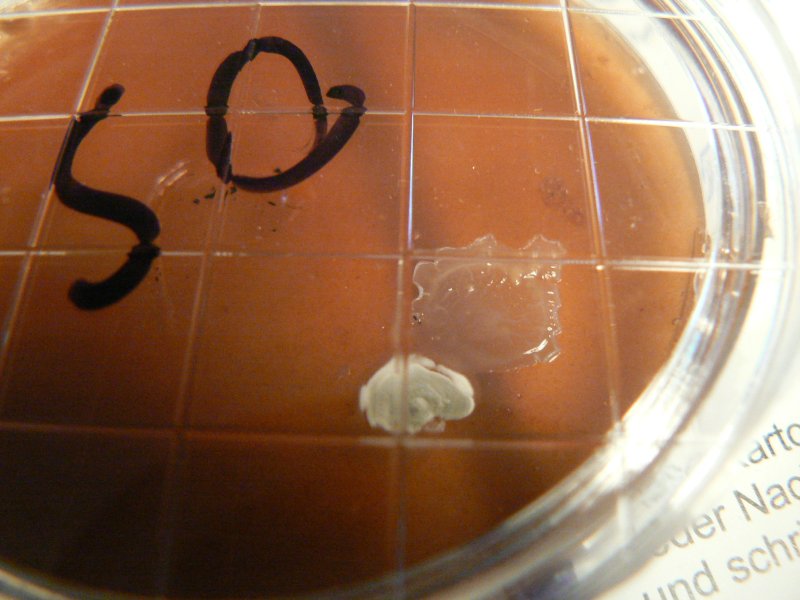
We used our ovamaltine/agartine mix as detailed below (perhaps with a bit more ovamaltine than yesterday). Dishes were labelled as OS (Ovamaltine/Spores).
As a further control the same recipe was used in two dishes (simply marked C) outside of any glove box on 20.11.11. They were left open in the room for perhaps 5 minutes while the agartine cooled.
Note: To prevent excess condensation in dishes which makes observation of specimens harder - allow to set with lid on and prior to inoculation wipe off excess moisture. Also recommended to stack dishes and avoid changes in temperature.
Results:
On 23.11.11 (4 days later) some growth is visible.
References:
http://www.mrcashop.org/mushroom_shop/index.php?content=Sporegermination
http://braukaiser.com/wiki/index.php?title=Inoculating_Plates_and_Slants
growing mycel with cloning
Use young mushrooms but where to find these at that time? In a shop. eg. enoki, oyster.
Agar recipes to test: MYA, PDYA (both using agartine from RUF which includes maltodextrin and maybe is too low in agar agar (20%)). Test also un-inoculated dishes.
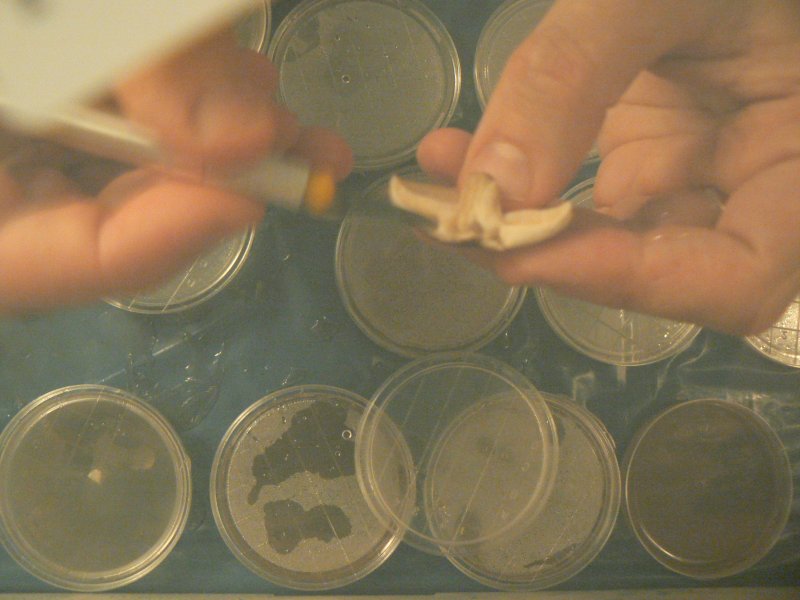
Clone piece from just above gills, unexposed flesh.
process followed:
- make agar mixtures and pour into sterilised dishes (in sterile environment “in the hood”)
- wait for agar to cool (in hood)
- flame sterilise scalpel
- cool tip by touching agar medium in petri dish
- tear mushroom apart and cut grain-sized sample
- quickly transfer into dish in same place as touched hot scalpel. insert part way into the medium
- seal dish
documentation:
Process above was followed with following notes:
Experiment conducted on 18.11.2011 using unidentified mushroom found growing in garden next to bulbous flowers. Also attempting to grow oyster mycelium on agar from inoculated wood plugs.
MEA was 2 cups water, 1 sachet agartine (20g) and 2 pinches ovamaltine.
PDYA was 1 cup water, 1 cup potato water, 2 piches yeast, 1 sachet agartine.
Marked as follows: first letter P=PDYA, O=ovamaltine; second letter U=unidentified, O=oyster mycelium, C=control, M=roots of found mushroom
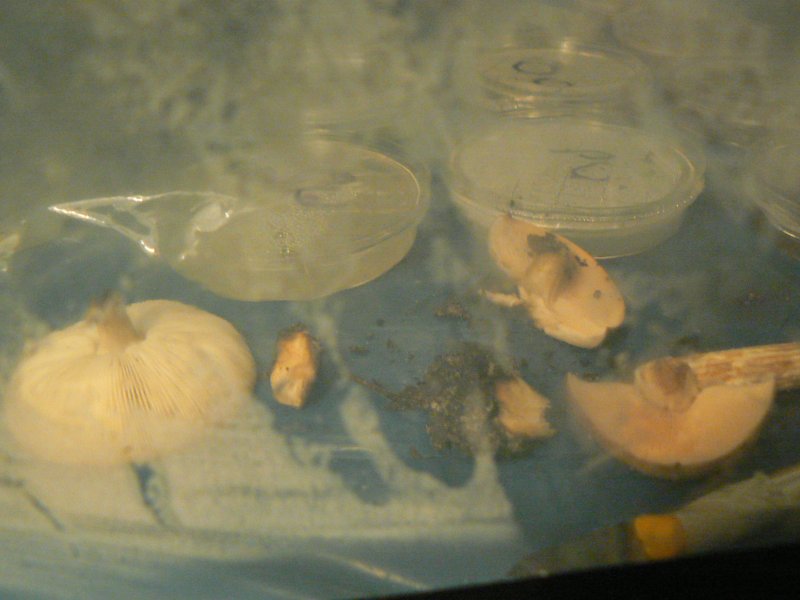
the unidentied mushroom to be cloned.
cutting an interior grain-sized portion with flame sterilised blade
Images with thanks to Laura Popplow.
Results 23.11.2011:
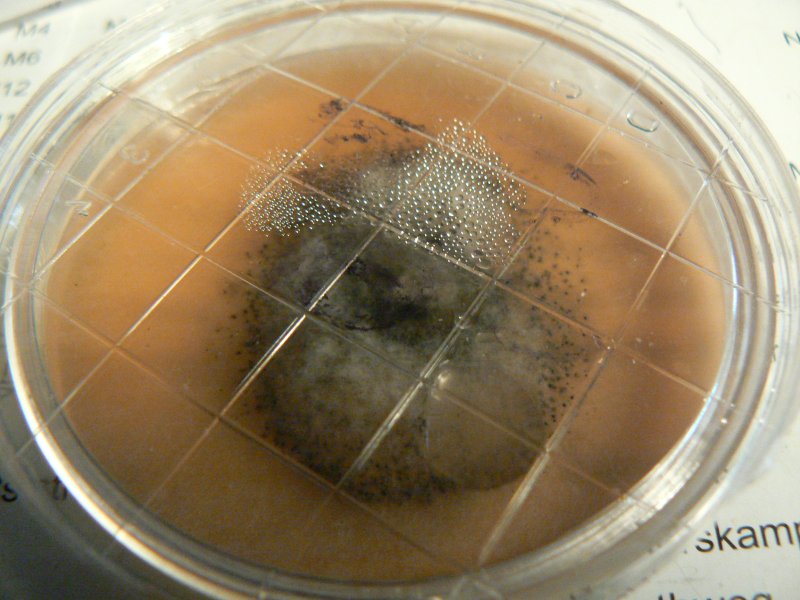
Unknown mushroom cloned on potato agar - vigorous growth of wispy white mould.
Soil and mycelium of unknown mushroom on potato agar.
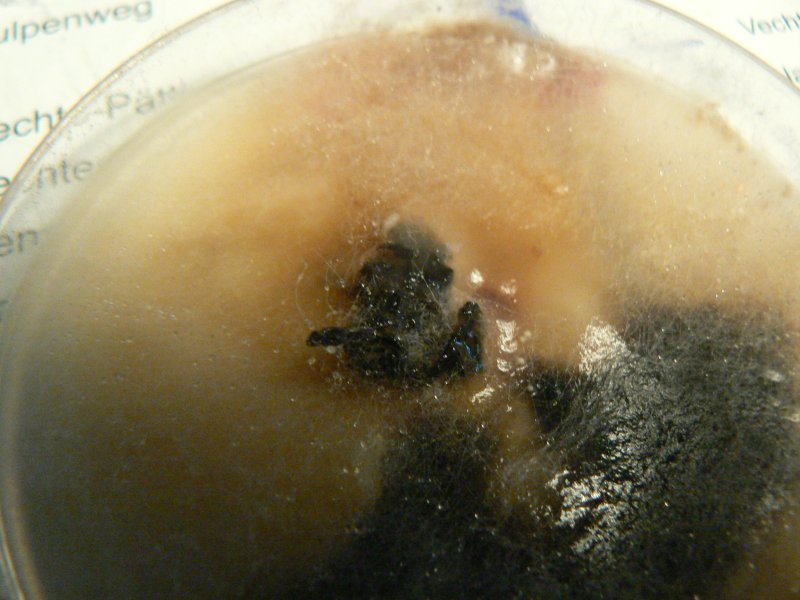
Mycelium from oyster inoculated plug on potato agar. Potato agar seems to support more vigorous growth of all fungi. Controls for potato agar shows the odd white speckle, for ovamaltine nothing so far.
Update 6.12.2011:
growing on a substrate
From either inoculated grain or inoculated wood plugs. First experiments started xx.xx.xx and 16.11.2011 growing oyster mushrooms (Pleurotus ostreatus) on various substrates including straw pellets, coffee grains and a mix of the two.
16.11.2011 experiments with coffee grounds
Coffee grounds from machine mixed with straw pellets and boiled water (experiment conducted by Laura Popplow). Inoculated with grain supplied by http://www.pilzmaennchen.de/
Coffee grounds from filter coffee machine. Inoculated with 3 wood plugs supplied by http://www.shii-take.de/
Coffee grounds on 5.12.2011 (moulds only!):
19.11.2011 experiments with magnetic tape (VHS)
Magnetic tape from a VHS videocassette (Blackfire on the German Geiselgasteig label) was unravelled into an empty juice tetrapac container (pierced with small holes). The container and tape were pasteurised with boiling water and six oyster inoculated wood plugs were placed within the curls of video tape. The whole ensemble was placed in a ziplock plastic bag and labelled. As a running control a juice container was filled with paper and coffee garins from the morning's filter brew with one inoculated plug inserted. More coffee and plugs will be inserted over the following days.
5.12.2011 fotokatier's spectral harvest
Measurement and intervention
mycelial transmitters
Transmissions/local emissions into mycelium:
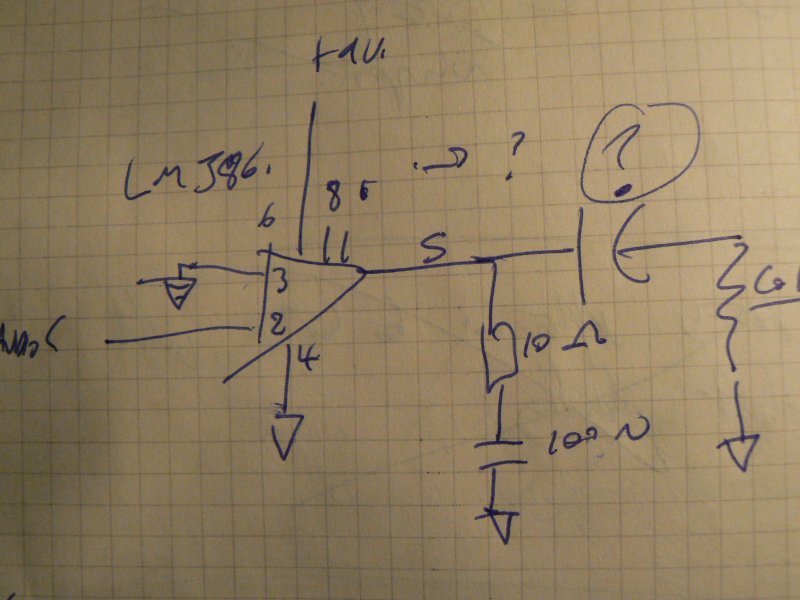
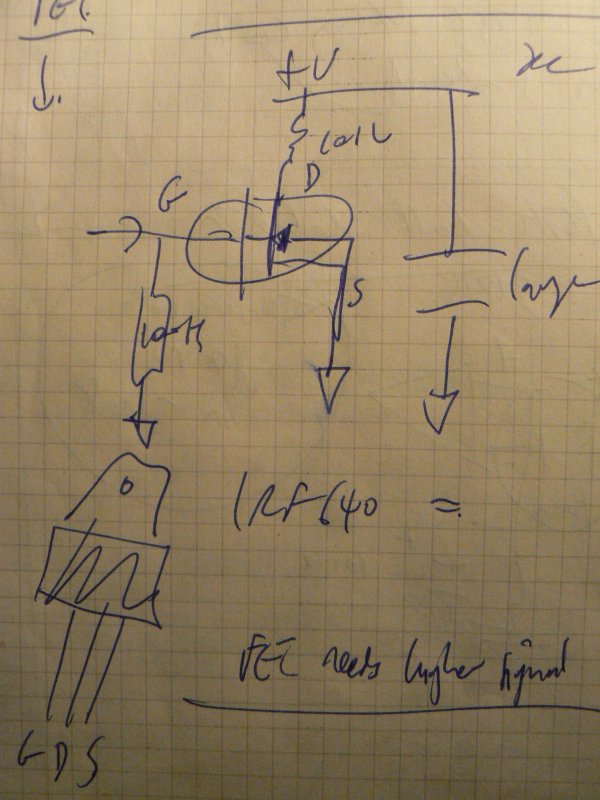
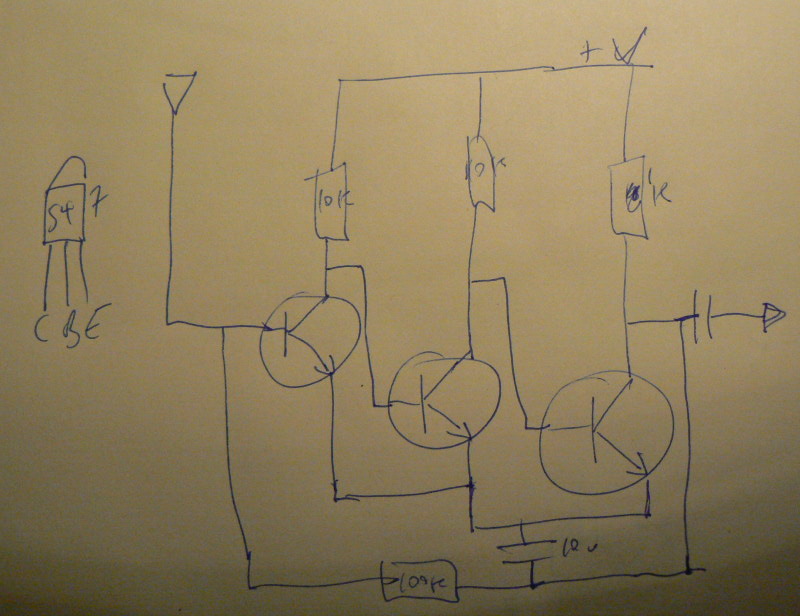
1] Local resonance amplifiers [LRA]:
Coil around the dish or mushroom, or coil (insulated) in the dish (how to exit the wires?)
Using LM386:
… or FET design
… to amplify local fields into the mycelium.
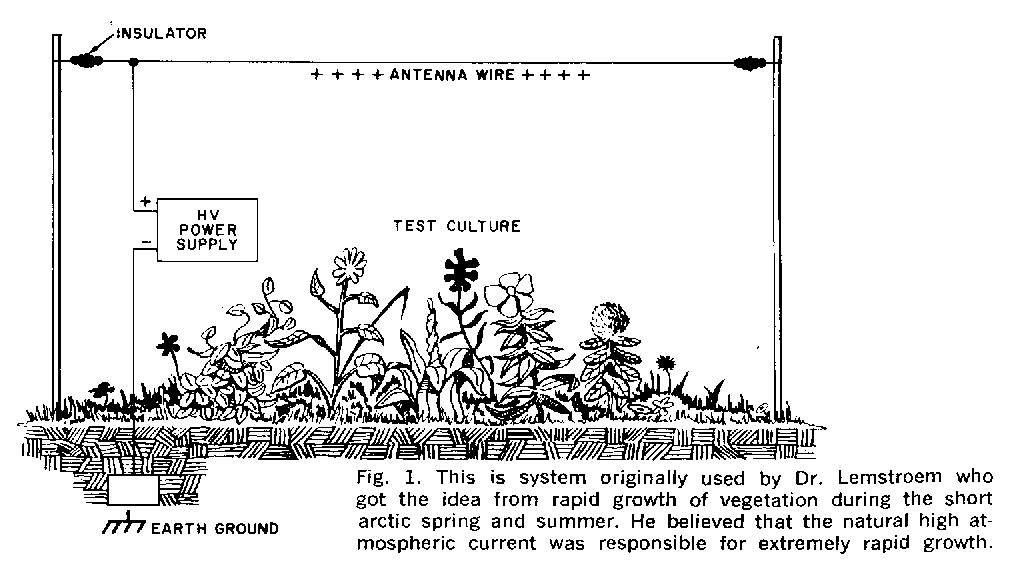
2] HV/electroculture
HV supply/potential on a wire above mycelium (need HV supply). Question of heating.
http://dwechsler.wordpress.com/2011/03/06/experimenting-with-electroculture-part1/
http://borderlandsofscience.blogspot.com/2010/07/more-experiments-in-electroculture.html
mycelial receivers
… receiving signals from the mycelium or mushroom/fungal body.
How to access tiny mycelia, some kind of micro wires aka. micro-electrodes?
Micro-electrodes appear to be silver wire which is etched and made smaller through electrolysis, these are embedded in a flame-shrunk micropipette which is then filled with electrolyte.
or an etched electrode in the bottom of the dish or container.
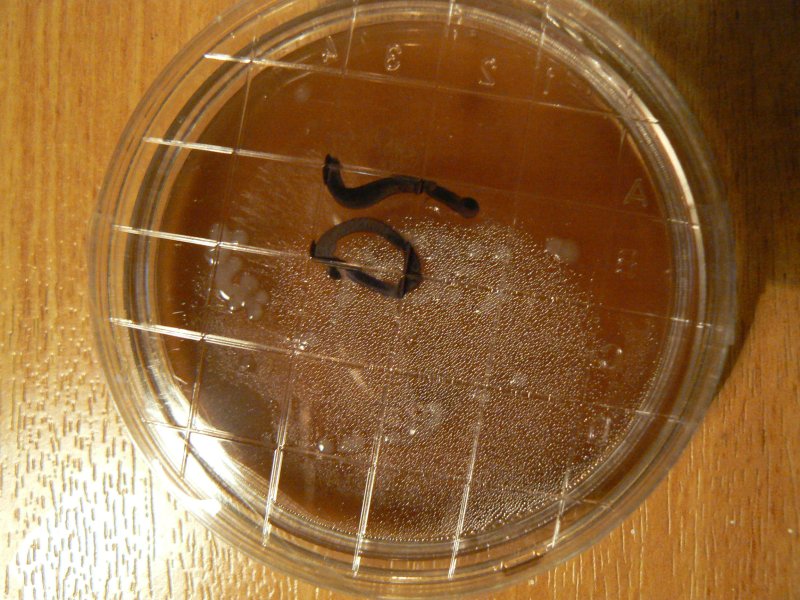
Experiments using copper tape (21.11.2011) stuck to bottom of dish. We could also somehow interface with mycelia? And a growing mushroom itself (on a tree trunk).
What is resistance of agar agar/agartine? Around 40-50,000 Ohms.
Do we need a faraday cage around our apparatus and how should we construct this? We could use a working or non-working microwave oven (but how to get signals for logging) or two foil covered cardboard boxes (grounded) inside one another.
1] GSR/wheatstone bridge
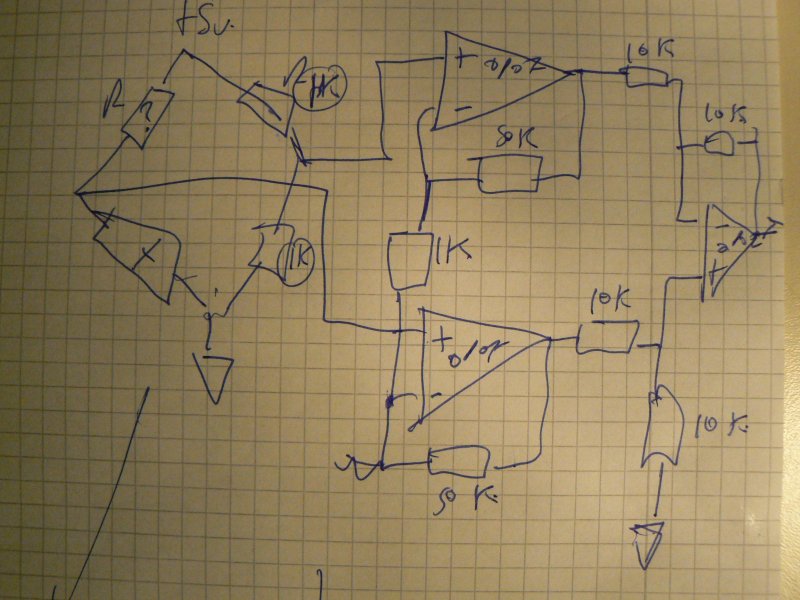
2] biopotential amplifier
With 3 electrodes - after psychotron on our http://www.1010.co.uk/org/biologic.html#sec-4 page.
3] EM field strength in/around the petri dish or mushroom (magnetometer, detector)
4] Look at bio-impedence
Using the AD5933 chip: so far results are inconclusive (also given low general resistance of the agartine mix)!
other/notes
Fungi and bio-luminescence (study using photomultiplier tubes, or simple photo-amp/photo-diodes).
Growing frequency-dependent fungi.
Refs:
http://www.mykoweb.com/articles/BioluminescentFungi.html
http://inamidst.com/lights/foxfire
Kaethe Wenzel recently mentioned a luminescent mould growing on cooked/left pasta.
making micro-electrodes
Notes
Amanita Muscaria
Growing Amanita (mycelium):
On PDYA (potato dextrose yeast agar), PDA or MYA (malt yeast agar). Also in liquid - grape juice mentioned.
References:
http://www.shroomery.org/forums/showflat.php/Number/6273949
https://mycotopia.net/forums/front-page-news/19812-amanita-cultivation-grape-juice.html
Mycelium
mycorrhiza: http://en.wikipedia.org/wiki/Mycorrhiza
A mycorrhiza is a symbiotic (generally mutualistic, but occasionally weakly pathogenic) association between a fungus and the roots of a vascular plant.
rhizomorphs are also called “Mycelial cords [which] are linear aggregations of parallel-oriented hyphae.” http://en.wikipedia.org/wiki/Mycelial_cord
basidiomycota - phyla. includes mushrooms, puffballs, stinkhorns, bracket fungi, other polypores, jelly fungi, boletes, chanterelles, earth stars, smuts, bunts, rusts, mirror yeasts, and the human pathogenic yeast Cryptococcus.
- different classifications of fungi:
mycorrhizal: symbiotic with roots of host plants (ecto and endomycorrhizal - outside and inside of roots)
parasitic
saprophytic: decomposers, wood-decomposing fungi. classified as primary, secondary and tertiary decomposers
hyphae branch and fuse to form multicellular network
network-wide response to differences and locations of nutrients
mycelial networks of hyphae connect two or more plants (colonised by the same mycorrhizas)
the network is the organism
rhizomorphs as specialised, high-conductivity organ (developed by saprotrophic and ectomycorrhizal basidiomycetes)
References:
Fungi in Ecosystem Processes By J. Dighton
Ecology of saprotrophic basidiomycetes By Lynne Boddy, Juliet C. Frankland, Pieter West, British Mycological Society
Soil Ecology in Northern Forests: A Belowground View of a Changing World By Martin Lukac, Douglas L. Godbold
Advances in mycorrhizal science and technology By Damase Khasa, Yves Piché, Andrew P. Coughlan
Adaptive Networks: Theory, Models and Applications By Thilo Gross, Hiroki Sayama (Adaptive biological networks…network development in mycelial fungi)
Future work
- frequency dependent fungi
- bio-luminescence as carrier wave for interspecies communication
- laptop petri-dish fungal portraits
- re-try with growing fungi on magnetic tape (dice up tape and use with straw pellets)
- similar process for integration with circuit board components (ground up) for The Crystal World